Accelerating Measurable Progress and Leveraging Investments for Postpartum Haemorrhage Impact (AMPLI-PPHI)
FIGO’s Accelerating Measurable Progress and Leveraging Investments for Postpartum Haemorrhage Impact (AMPLI-PPHI) project aims to dramatically reduce maternal mortality and morbidity from PPH. AMPLI-PPHI will bring catalytic change, connecting implementation learning with market shaping efforts, by demonstrating the feasibility, acceptability and cost effectiveness of a defined package of quality-assured PPH drugs – heat-stable carbetocin, tranexamic acid and misoprostol – within varying country contexts, at different levels of the health system, and within specific geographies.
Project contact
Rachel Gooden – Project Manager
The activities outlined in this project are supported by funding from Unitaid, innovating for global health.
The importance of accelerating progress and investments to reduce PPH impact
Severe bleeding after childbirth—postpartum haemorrhage (PPH)—is the leading cause of maternal mortality worldwide. The risk of PPH and PPH-related morbidity and mortality disproportionately affects women in low- and middle-income countries, especially those who lack access to quality care due to poverty, geography, or cultural barriers.
Heat-stable carbetocin (HSC) for the prevention of PPH, tranexamic acid (TXA) for the treatment of PPH, and misoprostol for advanced distribution to prevent PPH during community births could help change the trajectory of PPH-related mortality in these settings. However, until these drugs are accessible to countries, consistently available throughout supply chains, integrated into national clinical guidelines and trusted for use in real-world settings, the devastating consequences of PPH, especially in low-middle income countries, will continue.
What we do
FIGO is working as part of a consortium on this project, led by JHPIEGO and in partnership with PATH. Together, the AMPLI-PPHI consortium will directly support Ministries of Health to ensure that the right PPH drugs are available at the right time, in the right place, for the right indication and for the right patient across health systems, ultimately saving the lives of thousands of mothers and newborns.
This four-year (August 2022 - July 2026) Unitaid-funded project will support Ministries of Health in the Democratic Republic of Congo, Guinea, India and Kenya to integrate these drugs into clinical guidelines, generate evidence and learning to inform wide-scale implementation, and strengthen national health systems to procure and deliver quality-assured drugs to point of service. Further, beyond life of project, these efforts along with the relevant tools developed in target countries, will support the introduction and use of the three drugs in an additional 13 countries as well as additional states in India.
Project objectives
The project’s aims include:
- Generate evidence and project learning: Demonstrate feasibility, acceptability, cost-effectiveness and impact of three PPH drugs at all levels of the health system. Generate implementation and supply chain learning, as well as market intelligence.
- Create an enabling environment: Create a learning environment that will raise awareness, increase knowledge and build capacity among stakeholders at global, national and community levels. Disseminate best practices to influence countries through partnerships and regional champions.
- Prepare the market: Prime the market to achieve sustained availability and expanded access to quality-assured PPH drugs.
Introducing an integrated package of PPH in high-burden countries
FIGO and its partners Jhpiego and PATH, are working to catalyze the early adoption and scale-up of new and recently recommended drugs to prevent or treat PPH in high-burden countries as part of an integrated package of PPH. This includes tranexamic acid (TXA) (treatment of PPH), heat-stable carbetocin (HSC) (Prevention of PPH during AMTSL in facilities) and misoprostol (Prevention of PPH during community births through advance distribution to women for self-administration) to be used as tools to reduce the impact of PPH.
Project details
This project will run for 4 years (August 2022 - July 2026) and is funded by Unitaid.
We are working through our member societies in Democratic Republic of Congo, Guinea, India and Kenya and will engage an additional 13 member societies as well as additional States in India, to share the learning and knowledge that is generated, for wider dissemination and to support on-going PPH efforts.
Our current partners include:
- Kenya Obstetrical Gynaecological Society (KOGS)
- Société Guinéenne de Gynécologie -Obstétrique (SGGO)
- Société Congolaise de Gynécologie et d’Obstétrique (SCOGO)
- The Federation of Obstetric and Gynaecological Societies of India (FOGSI)
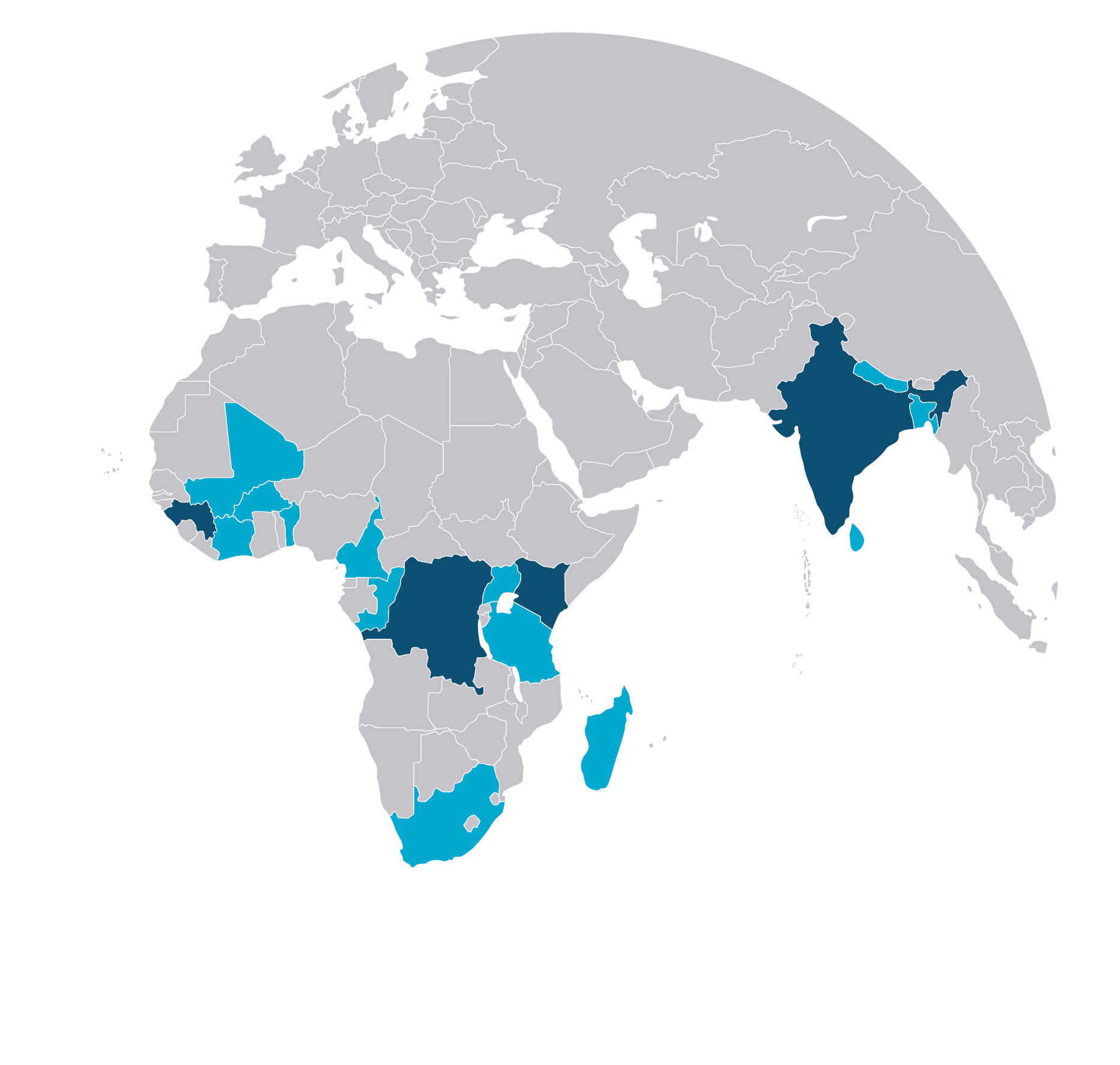
Project countries: Democratic Republic of the Congo, Guinea, Kenya and India
Learning exchange countries: Madagascar, Congo-Brazzaville, Cameroon, Burkina Faso, Benin, Côte d’Ivoire, Mali, Bangladesh, Nepal, India (five additional states), Sri Lanka, Tanzania, Uganda and South Africa

